Extracellular vesicles (EVs) are small ‘delivery trucks’ released by our cells that deliver important materials to other cells to aid cellular communication. This study revealed, for the first time, a link between levels of EVs in the blood and tissue damage caused by diseases such as leukaemia.
Researchers hope to leverage the critical new insight to develop a blood test to monitor cancer patients with tissue damage, which could, in future, enhance treatment strategies for blood cancers and other diseases.
Extracellular vesicles (EVs) are like small delivery trucks that are dispatched by our cells to distribute important materials like proteins, fats and genetic information to other cells.
This delivery system helps cells communicate with each other, especially when they are under stress or dying.
Research into how EVs form and their link to disease progression is challenging because of their small size, with most studies restricted to a ‘cells-in-a-dish’ approach.
In an unprecedented study, researchers were able to overcome this significant barrier by imaging live EVs inside the bone marrow of mice.
The study involved a significant collaboration with Professor Ivan Poon, Director of the La Trobe Research Centre for Extracellular Vesicles (RCEV) – the largest group of EV researchers in the Southern Hemisphere.
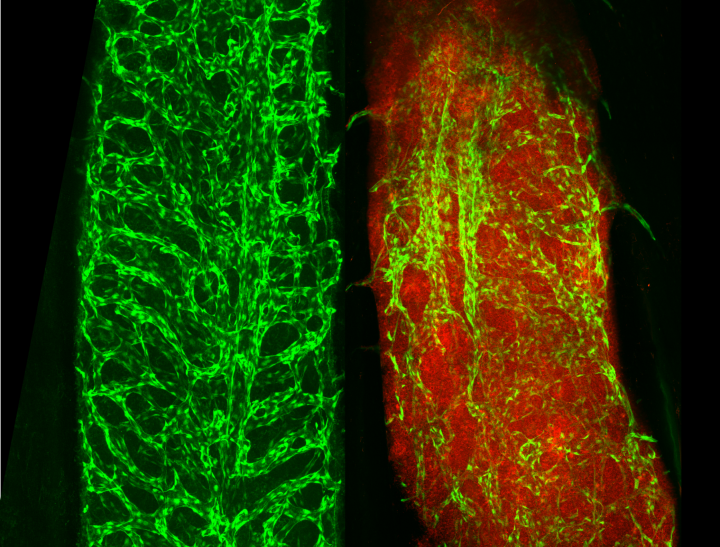
La Trobe PhD candidate and second author, Jascinta Santavanond, said the La Trobe team was able to capture and monitor biological processes occurring in the vasculature of living zebrafish, using the world-class facilities of the La Trobe University Bioimaging Platform.
“This study is exciting as it may help with the development of new diagnostic tools. Specifically, the levels of endothelial cell extracellular vesicles found in the blood could be used as an indicator of the level of the amount of tissue damage observed during disease, including blood cancers such as leukemia,” she said.
“By working together, we have validated our results in fish, mice and human samples, truly highlighting the impact of the research.”
First author and WEHI cell biologist, Dr Georgia Atkin-Smith, said the team used high-resolution microscopy that can see directly inside the bone marrow of live organisms to capture the formation of EVs from blood vessels.
“No other study in the world has been able to achieve this so it’s a huge win for Australia’s scientific community,” Dr Atkin-Smith said.
“In this study, we’ve shown that the development of leukemia can degrade healthy blood vessels in the bone marrow. Mice with extensive blood vessel damage in their bone marrow had elevated levels of EVs in their blood, while healthy mice did not.
“This revealed – for the first time – that there is a link between EVs in the blood and tissue damage during cancer.
The potential link between EVs and blood vessel damage was first hypothesised in 2018 by senior author and WEHI Laboratory Head, Associate Professor Edwin Hawkins, who is an expert in ‘in vivo imaging’ – techniques that allow researchers to see inside living organisms.
“To have seen how the process of EV formation occurs with our own eyes after four years of research was an incredible moment,” Dr Atkin-Smith said.
“Pictures tell a thousand words, and these ones have significantly advanced our understanding into EVs by showing how they form under both healthy settings and during disease.
“This has not only developed a new framework to study the formation of EVs in model organisms, but could inform new diagnostic tools to monitor the level of tissue damage observed during disease through a simple blood test. It’s an incredibly exciting advancement.”
EV biology: From fish to mice to humans
The WEHI research team is now assessing whether EVs can be used as a biomarker in acute myeloid leukemia (AML) patients, through a collaboration with the Peter MacCallum Cancer Centre (Peter Mac). They hope to develop new tools and techniques that would allow clinicians to determine the impact of disease on healthy tissue, and assess the disease progression by analysing patient samples.
The study, published in Nature Communications, also involved collaborations with the University of Melbourne, The Florey, Olivia Newton-John Cancer Research Institute, Peter Mac and Monash University.
The research was supported by the National Health and Medical Research Council, Australian Research Council, CASS Foundation, Jack Brockhoff Foundation, L’Oreal UNESCO For Women in Science, Victorian Cancer Agency, a Sir Clive McPherson Family Fellowship and a Rae Foundation grant.
Image: Blood vessels (pictured in green) in healthy bone marrow; blood vessels in bone marrow that has AML (pictured in red).
Credit: WEHI